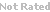 Loading... Please wait...
Loading... Please wait...Free Delivery Order Amount: HK Island/New Territories $1500; Shatin/Tsuen Wan/Kwai Ching $1200; Kowloon $1000; HC Self Pick-up Point $600
- Home
- Color Stain & Raw Materials
- Colouring Oxides
- Titanium Dioxide (Anatase, Brookite)
Categories
Our Newsletter
Product Description
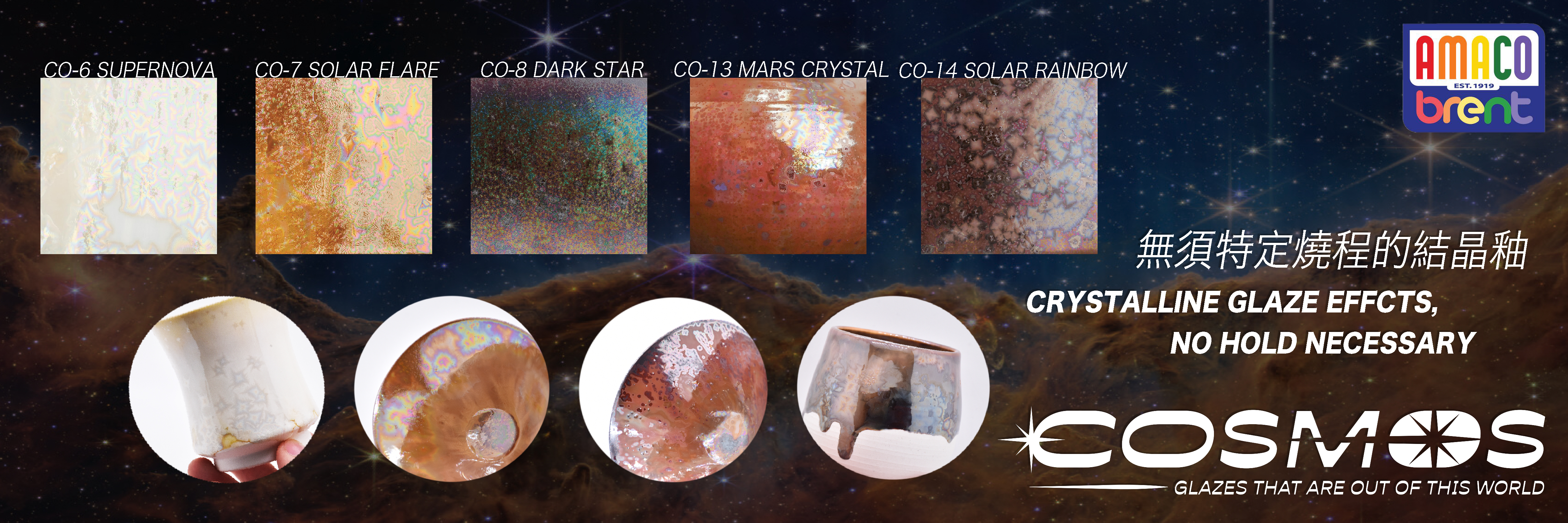
-Titania by itself is quite refractory. But when other oxides are present in the melt TiO2 becomes much more complex: because it opacifies, variegates, and crystallizes glazes. It also modifies existing colors from metals like Cr, Mn, Fe, Co, Ni, Cu.
-In amounts below 1% titania can dissolve completely in a glaze melt. In slightly greater amounts it can give a bluish-white flush to transparent glazes (depending on their amount of alumina).
-Above 2% it begins to significantly alter the glaze surface and light reflectance properties through the creation of minute crystals. This crystal mechanism gives soft colors and pleasant opacity, and breaks up and mottles the surface. In the 2-6% range, it increasingly variegates the glaze surface. Many potters add titania to their glazes or paint on overglaze titania washes for this purpose.
-As amounts increase above 5% the opacity and matteness accelerates. As much as 25% can be absorbed by some lead glazes. Up to 0.8 molar can be used to effect crystal melts in glossy glazes.
-Although titania will form a glass by itself, it is not highly soluble in silica melts. However, it is considered by some as a glass former in certain circumstances since it can stiffen the melt and stabilize the fired glass against leaching (i.e. it is used in lead frits to lessen the solubility of the lead).
-Titania can act as a modifier and within a narrow range it will combine with fluxes to make a glass. It can also act in a flux-like way in very high silica melts.
-Minute amounts (e.g. 0.1%) can be used to intensify and stabilize colors (i.e. iron can be altered to produce yellow and orange). In small amounts (e.g. 1%) it can alter and intensify existing color and opacity in a glaze.
-Titania can be reduced to produce colors in keeping with the elements present. If highly reduced it can yield a red, with iron the color could be yellow, brown or green. Other combinations can yield blues, greens, yellows. Titania is oxygen-hungry and will quickly oxidize from its reduced state if given the chance.
-Glazes containing titania are phototropic and can change color slightly by the action of light. They can also be thermotropic in that they can change color (i.e. toward yellow) when heated.
-Titanium frits are used to opacify and whiten engobes in the production of ceramic tile.
-Some have chosen to treat TiO2 as an 'inert' with respect to the chemistry of the glaze. However, a phase diagram of Al2O3 and TiO2 shows a eutectic at 80% Al2O3 at 1705C demonstrating that TiO2 does 'react' with the second most important ceramic oxide.
-TiO2 is considered an impurity in ball clays and kaolins used to make porcelain because it can react with any iron present to form rutile crystals which detrimentally affect body color and tranlucency.
You Recently Viewed...
New Products
-
$205.00
-
$105.00$94.50
-
$212.00$190.80
-
$275.00$247.50
-
$65.00$58.50